I got asked if USING anodes, in an engine is a good idea?
electrolysis can rapidly cause damage, use of a 50% anti-freeze/50% water coolant mix and at least two good separate engine grounds to the cars frame helps but get out a multi meter and test for voltage in coolant, (more info in links)
any time you use aluminum heads on an iron block (ESPECIALLY WITH COPPER HEAD GASKETS )
but any head gasket on an iron block with aluminum heads, or an aluminum intake, or aluminum, water pump,
is going to have issues over time,
your forced to use ANODES and replace those ANODES regularly as they are designed to sacrificially corrode rather than the more expensive components, and replace the anti freeze at least every 12-18 months or the result you got (parts in contact with coolant_ corrosion,) is very common
Sacrificial anodes are used to protect metal structures from corroding. Sacrificial anodes work by oxidizing more quickly than the metal it is protecting, being consumed completely before the other metal reacts with the electrolytes.
ITS NOT ONLY A GOOD IDEA
ITS MANDATORY IN SOME APPLICATIONS!
if you were for example to use
aluminum heads
on a cast iron block
with a copper head gasket
(something IVE done frequently)
its MANDATORY,
if you use an ALUMINUM AFTERMARKET RADIATOR ITS MANDATORY,
IF YOU USE AN ALUMINUM WATER PUMP<
ITS MANDATORY, to use ANODES FOR DURABILITY , ANY USE OF DIS SIMILAR METALS MAKES IT MANDATORY that you use anodes and a 50% antifreeze coolant solution to significantly reduce electrolysis and the damage it WILL CAUSE!
you need to realize you've effectively built a battery if you don,t use both several ANODES and at least a 50% or higher concentration of anti freeze as the dissimilar metals will cause the aluminum, to disintegrate over time without the anodes and high anti freeze concentration.
Ive been building engines for over 40 years and yes I fully agree some head gaskets suggest installing them dry....I spray both sides wet with copper coat and have yet to have problems with them sealing, keep in mind the vast majority of head gaskets don,t "LEAK" they get damaged by "OVER HEATING" or "DETONATION DAMAGED" or " effected by coolant corrosion" then when they fail, its blamed on the "HEAD GASKET FAILURE" THATS A BIT LIKE BLAMING FLYS for CAUSING GARBAGE
I rarely use anything BUT dead soft pure copper head gaskets sprayed damp, with copper coat spray, on both sides, installed on my engines, Ive YET to have any leak in over 18 years Ive used them
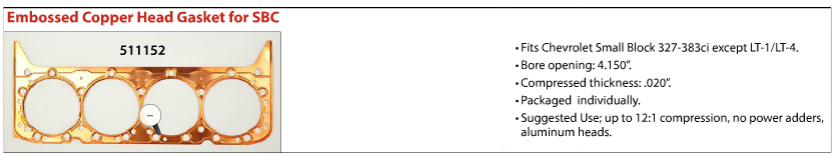
now these embossed copper gaskets (above) are supposed to be the best choice, but Ive never seen or used those
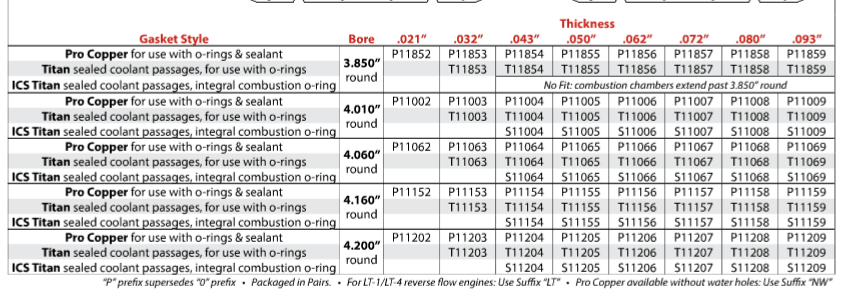
these flat copper gaskets are supposed to be used with a wire fire ring with a groove machined into the block,Ive been successfully using these gaskets

without those for decades by just spraying them damp with copper coat and have never yet had one fail

when installing almost any head gasket, but especially with a copper head gasket be sure to put on two wet even coats on both sides of the head gasket and install and torque while the copper coat sprays still damp
(mostly because they are re-usable after a good cleaning) and I pull the engine apart frequently for cam swaps and other mods
if you use a copper head gasket on an iron block with aluminum heads without anodes youll have severe corrosion issues
http://www.summitracing.com/parts/sdk-c ... 7AodUncAfw
http://www.martyranodes.com/content/mar ... anodes.php
http://www.rotometals.com/E-Series-E-0- ... s-s/65.htm
http://www.flex-a-lite.com/accessories/ ... anode.html
https://www.cgj.com/2013/07/02/aluminum ... ptibility/
http://garage.grumpysperformance.co...ow-to-reduce-its-likely-hood.9816/#post-49741
https://www.amazon.com/Northern-Z17700-Sacrificial-Anode-Rad/dp/B005ASS0NQ

ITS ALMOST MANDATORY on cars with aluminum heads on an iron engine block and an aluminum radiator.
IF you pull your intake and find its starting to corrode badly in the coolant ports, or your aluminum heads are ,as well,that's an indication of a problem. usually the result of a bad engine ground or anti-freeze that needs to be changed out every 2 years MINIMUM, or an electrical problem.
that's far more common on BOATS, especially those used in salt water or where the battery grounds attached to an intake bolt, but in cars its usually a sign of a BAD ENGINE GROUND and LACK of a RADIATOR ANODE, and running just water as a coolant vs ANTIFREEZE & COOLANT in the radiator, ideally in about a 50%/50% mix .Antifreeze REDUCES cooling efficiency slightly compared to distilled water but it significantly reduces corrosion
Antifreeze has a high viscosity so its flow rate is less than water and it doesn't transfer heat as well either.
A 50/50 mix is just a compromise between cooling efficiency and frozen or corrosion in your engine block, heads or radiator.
one other factor often over looked is the anti-freeze concentration, your going to ideally use a 50% water 50% antifreeze mix that balances cooling efficiency with corrosion resistance, if you run strait water electrolysis is very likely, almost certainly going to cause major problems, but if you run strait anti-freeze you'll run 15-20 degrees hotter as strait anti-freeze does not transfer heat as effectively BTW, A BAD GROUND can also cause the pilot bearing on a manual trans to show damage IF both the trans and engine are not grounded to the frame and the battery to the same frame
heres an old post
I got asked if anodes are a good idea?
well, when you run an aluminum performance cylinder head on an iron engine block with pure copper head gaskets, and aluminum radiator ,like Ive been doing for many years, those anodes are mandatory, and yes they do work and prevent or at least slow electrolysis a great deal
naturally you'll need to use the anodes in the block vs the radiator with a plastic radiator like some cars have, and if you use components like an iron block, aluminum heads and copper head gaskets use of several anodes is MANDATORY
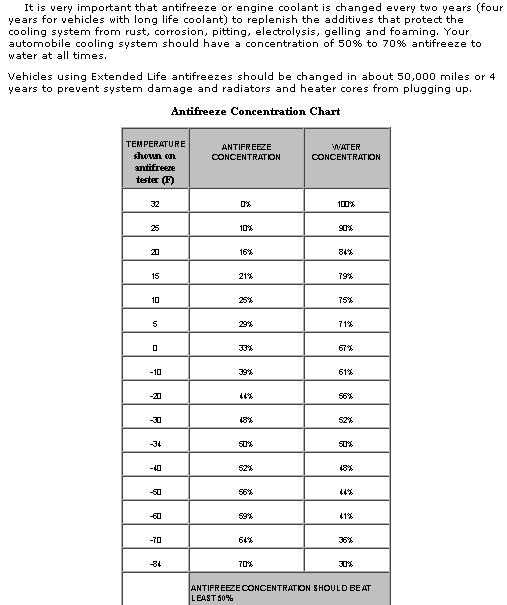

http://www.ve-labs.net/electrolysis-101/how-to-test
http://www.performancemetals.com/anodes/AnodeFAQs.shtml
http://www.flex-a-lite.com/accessories/ ... anode.html
http://www.bmcno.org/RadCap.htm
http://www.jcwhitney.com/autoparts/...10101&sku=anode&searchbtn.x=16&searchbtn.y=12
viewtopic.php?f=57&t=9769
anodes are easy to find at larger marine supply stores and you can cut some to fit.
https://www.westmarine.com/engine-anodes
https://www.amazon.com/Northern-Z17...coding=UTF8&psc=1&refRID=DRQX2RHJS9100ZF2H8CH
http://www.boatzincs.com/engine.html
http://www.boatzincs.com/engine-siz...MIjc7mjeyv2wIVBQaGCh1rCgq3EAQYASABEgIIpvD_BwE
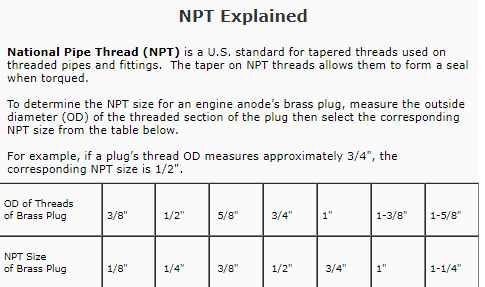
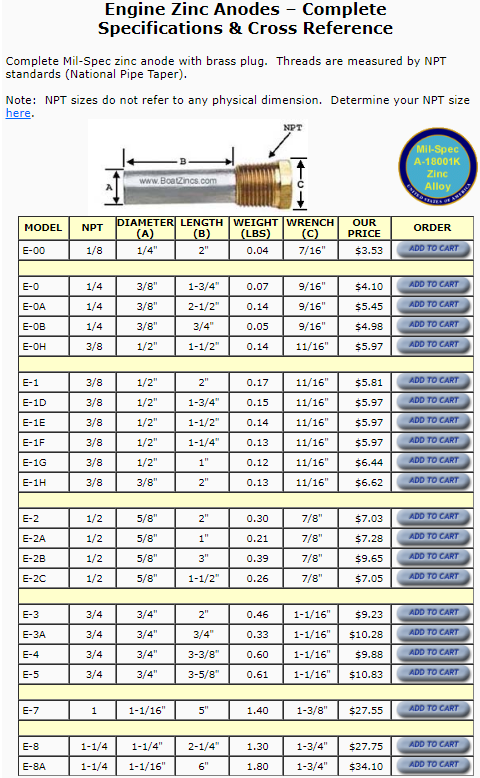
IDEALLY theres ZERO MEASURABLE voltage! and with both a decent frame to engine ground and battery to frame ground theres seldom much voltage in the coolant, but having a transmission to frame ground in addition is a good idea as Ive seen that help also, a loose ground causes lots of flaky/intermitent problems
Determining the levels of EME present in the cooling system is very easy. All you really need is an ultrasensitive voltmeter. Simply remove the radiator cap, stick the positive probe into the cooling system, and ground the negative probe to the radiator. With the meter dialed down to the lowest voltage setting, take a reading of the total amount of electrical volts present in the cooling system. The reading should be somewhere BELOW 0.01 volts, if its not you NEED to find out where the grounds loose or an extra grounds needed and add an anode
running low anti-freeze concentrations like nearly all water,hurts the engine also, especially if its not distilled like from a hose where its got a higher mineral content so it conducts current better.
ANTI FREEZE needs to be replaced every 2 years or so and the RADIATOR FLUSHED of the crud that tends to accumulate, to keep it free of the stuff that tends to settle out and clog the passages. and to prevent the water acting like an electrolyte in a battery.
adding an ANODE can help.
viewtopic.php?f=57&t=74
COOLING SYSTEM ZINC ANODE, FITS IN RADIATOR IN PLACE OF PETCOCK
the ORANGE and GREEN anti-freeze most of us use are sometimes NOT compatible and if blended , they over time form a sludge that clogs the passages, THEY SHOULD NEVER BE MIXED
adding an ANODE tends to slow the Corrosion, and having a good engine ground also tends to prevent or at least delay freeze plug and radiator corrosion problems.
electrolysis can rapidly cause damage, use of a 50% anti-freeze/50% water coolant mix and at least two good separate engine grounds to the cars frame helps but get out a multi meter and test for voltage in coolant, (more info in links)
any time you use aluminum heads on an iron block (ESPECIALLY WITH COPPER HEAD GASKETS )
but any head gasket on an iron block with aluminum heads, or an aluminum intake, or aluminum, water pump,
is going to have issues over time,
your forced to use ANODES and replace those ANODES regularly as they are designed to sacrificially corrode rather than the more expensive components, and replace the anti freeze at least every 12-18 months or the result you got (parts in contact with coolant_ corrosion,) is very common
Sacrificial anodes are used to protect metal structures from corroding. Sacrificial anodes work by oxidizing more quickly than the metal it is protecting, being consumed completely before the other metal reacts with the electrolytes.
ITS NOT ONLY A GOOD IDEA
ITS MANDATORY IN SOME APPLICATIONS!
if you were for example to use
aluminum heads
on a cast iron block
with a copper head gasket
(something IVE done frequently)
its MANDATORY,
if you use an ALUMINUM AFTERMARKET RADIATOR ITS MANDATORY,
IF YOU USE AN ALUMINUM WATER PUMP<
ITS MANDATORY, to use ANODES FOR DURABILITY , ANY USE OF DIS SIMILAR METALS MAKES IT MANDATORY that you use anodes and a 50% antifreeze coolant solution to significantly reduce electrolysis and the damage it WILL CAUSE!
you need to realize you've effectively built a battery if you don,t use both several ANODES and at least a 50% or higher concentration of anti freeze as the dissimilar metals will cause the aluminum, to disintegrate over time without the anodes and high anti freeze concentration.
Ive been building engines for over 40 years and yes I fully agree some head gaskets suggest installing them dry....I spray both sides wet with copper coat and have yet to have problems with them sealing, keep in mind the vast majority of head gaskets don,t "LEAK" they get damaged by "OVER HEATING" or "DETONATION DAMAGED" or " effected by coolant corrosion" then when they fail, its blamed on the "HEAD GASKET FAILURE" THATS A BIT LIKE BLAMING FLYS for CAUSING GARBAGE
I rarely use anything BUT dead soft pure copper head gaskets sprayed damp, with copper coat spray, on both sides, installed on my engines, Ive YET to have any leak in over 18 years Ive used them

now these embossed copper gaskets (above) are supposed to be the best choice, but Ive never seen or used those

these flat copper gaskets are supposed to be used with a wire fire ring with a groove machined into the block,Ive been successfully using these gaskets

without those for decades by just spraying them damp with copper coat and have never yet had one fail

when installing almost any head gasket, but especially with a copper head gasket be sure to put on two wet even coats on both sides of the head gasket and install and torque while the copper coat sprays still damp
(mostly because they are re-usable after a good cleaning) and I pull the engine apart frequently for cam swaps and other mods
if you use a copper head gasket on an iron block with aluminum heads without anodes youll have severe corrosion issues
http://www.summitracing.com/parts/sdk-c ... 7AodUncAfw
http://www.martyranodes.com/content/mar ... anodes.php
http://www.rotometals.com/E-Series-E-0- ... s-s/65.htm
http://www.flex-a-lite.com/accessories/ ... anode.html
https://www.cgj.com/2013/07/02/aluminum ... ptibility/
http://garage.grumpysperformance.co...ow-to-reduce-its-likely-hood.9816/#post-49741
https://www.amazon.com/Northern-Z17700-Sacrificial-Anode-Rad/dp/B005ASS0NQ
ITS ALMOST MANDATORY on cars with aluminum heads on an iron engine block and an aluminum radiator.
IF you pull your intake and find its starting to corrode badly in the coolant ports, or your aluminum heads are ,as well,that's an indication of a problem. usually the result of a bad engine ground or anti-freeze that needs to be changed out every 2 years MINIMUM, or an electrical problem.
that's far more common on BOATS, especially those used in salt water or where the battery grounds attached to an intake bolt, but in cars its usually a sign of a BAD ENGINE GROUND and LACK of a RADIATOR ANODE, and running just water as a coolant vs ANTIFREEZE & COOLANT in the radiator, ideally in about a 50%/50% mix .Antifreeze REDUCES cooling efficiency slightly compared to distilled water but it significantly reduces corrosion
Antifreeze has a high viscosity so its flow rate is less than water and it doesn't transfer heat as well either.
A 50/50 mix is just a compromise between cooling efficiency and frozen or corrosion in your engine block, heads or radiator.
one other factor often over looked is the anti-freeze concentration, your going to ideally use a 50% water 50% antifreeze mix that balances cooling efficiency with corrosion resistance, if you run strait water electrolysis is very likely, almost certainly going to cause major problems, but if you run strait anti-freeze you'll run 15-20 degrees hotter as strait anti-freeze does not transfer heat as effectively BTW, A BAD GROUND can also cause the pilot bearing on a manual trans to show damage IF both the trans and engine are not grounded to the frame and the battery to the same frame
heres an old post
I got asked if anodes are a good idea?
well, when you run an aluminum performance cylinder head on an iron engine block with pure copper head gaskets, and aluminum radiator ,like Ive been doing for many years, those anodes are mandatory, and yes they do work and prevent or at least slow electrolysis a great deal
naturally you'll need to use the anodes in the block vs the radiator with a plastic radiator like some cars have, and if you use components like an iron block, aluminum heads and copper head gaskets use of several anodes is MANDATORY

http://www.ve-labs.net/electrolysis-101/how-to-test
http://www.performancemetals.com/anodes/AnodeFAQs.shtml
http://www.flex-a-lite.com/accessories/ ... anode.html
http://www.bmcno.org/RadCap.htm
http://www.jcwhitney.com/autoparts/...10101&sku=anode&searchbtn.x=16&searchbtn.y=12
viewtopic.php?f=57&t=9769
anodes are easy to find at larger marine supply stores and you can cut some to fit.
https://www.westmarine.com/engine-anodes
https://www.amazon.com/Northern-Z17...coding=UTF8&psc=1&refRID=DRQX2RHJS9100ZF2H8CH
http://www.boatzincs.com/engine.html
http://www.boatzincs.com/engine-siz...MIjc7mjeyv2wIVBQaGCh1rCgq3EAQYASABEgIIpvD_BwE


IDEALLY theres ZERO MEASURABLE voltage! and with both a decent frame to engine ground and battery to frame ground theres seldom much voltage in the coolant, but having a transmission to frame ground in addition is a good idea as Ive seen that help also, a loose ground causes lots of flaky/intermitent problems
Determining the levels of EME present in the cooling system is very easy. All you really need is an ultrasensitive voltmeter. Simply remove the radiator cap, stick the positive probe into the cooling system, and ground the negative probe to the radiator. With the meter dialed down to the lowest voltage setting, take a reading of the total amount of electrical volts present in the cooling system. The reading should be somewhere BELOW 0.01 volts, if its not you NEED to find out where the grounds loose or an extra grounds needed and add an anode
running low anti-freeze concentrations like nearly all water,hurts the engine also, especially if its not distilled like from a hose where its got a higher mineral content so it conducts current better.
ANTI FREEZE needs to be replaced every 2 years or so and the RADIATOR FLUSHED of the crud that tends to accumulate, to keep it free of the stuff that tends to settle out and clog the passages. and to prevent the water acting like an electrolyte in a battery.
adding an ANODE can help.
viewtopic.php?f=57&t=74
COOLING SYSTEM ZINC ANODE, FITS IN RADIATOR IN PLACE OF PETCOCK
the ORANGE and GREEN anti-freeze most of us use are sometimes NOT compatible and if blended , they over time form a sludge that clogs the passages, THEY SHOULD NEVER BE MIXED
adding an ANODE tends to slow the Corrosion, and having a good engine ground also tends to prevent or at least delay freeze plug and radiator corrosion problems.
Attachments
Last edited by a moderator:






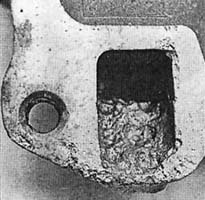










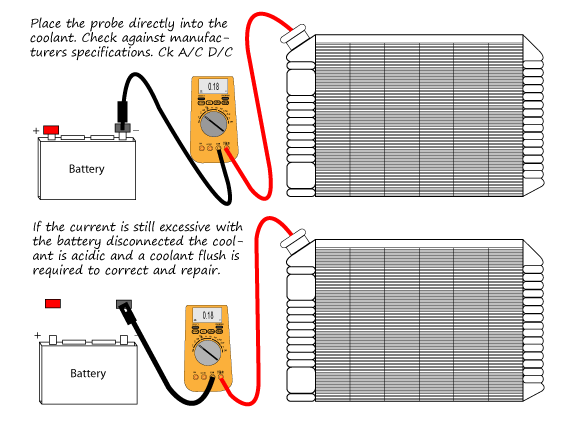

 while in an ideal world the anode would be screwed into the lower radiator housing, from a conductivity the sacrificial properties and function would not be affected.
while in an ideal world the anode would be screwed into the lower radiator housing, from a conductivity the sacrificial properties and function would not be affected.