ANY ANTI FREEZE needs to be replaced every 3-5 years or so or at 150K miles ,AS A MAXIMUM LIFE EXPECTANCY, MOST LOSE SIGNIFICANT LEVELS OF EFFECTIVE CORROSION PROTECTION GRADUALLY FROM 24 MONTHS and about 40,000 miles and its a good idea to have the RADIATOR FLUSHED of the crud that tends to accumulate, too keep it free of the stuff that tends to settle out and clog the passages. and too prevent the water / coolant mix from breaking down and acting like an electrolyte in a battery, in an ideal world youll want to replace antifreeze with a 50%/50% mix of antifreeze and distilled water,one other factor often over looked is the anti-freeze concentration, your going to ideally use a 50% water 50% antifreeze mix that balances cooling efficiency with corrosion resistance, if you run strait water electrolysis is very likely, almost certainly going to cause major problems, but if you run strait anti-freeze youll run 15-20 degrees hotter as strait anti-freeze does not transfer heat as effectively as water or a 50%/50% mix.
adding an ANODE can help, reduce corrosion .

http://www.radiator.com/article-radiator-problems.html
http://www.radiatorinfo.com/radtip.html
http://www.aa1car.com/library/cooling_s ... rosion.htm
viewtopic.php?f=57&t=9769
http://www.sancarlosradiator.com/antifreeze_coolant.htm
http://forum.grumpysperformance.com/viewtopic.php?f=57&t=74
http://www.ehow.com/how_2189727_prevent-clogged-radiator.html
http://automechanics.wordpress.com/2008/05/28/top-ten-common-radiator-problems-for-older-vehicles/
the ORANGE and GREEN anti-freeze most of us use are sometimes NOT compatible and if blended , IF mixed they frequently over time form a sludge, or break down and leave crud in the radiator passages that clogs the passages, THEY SHOULD NEVER BE MIXED
https://durathermfluids.com/pdf/techpapers/pressure-boiling-point.pdf
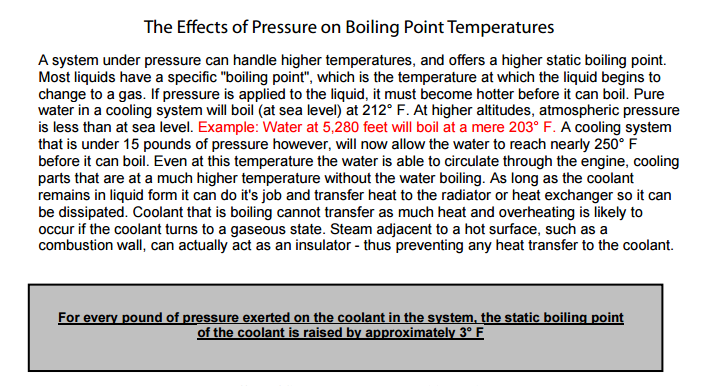
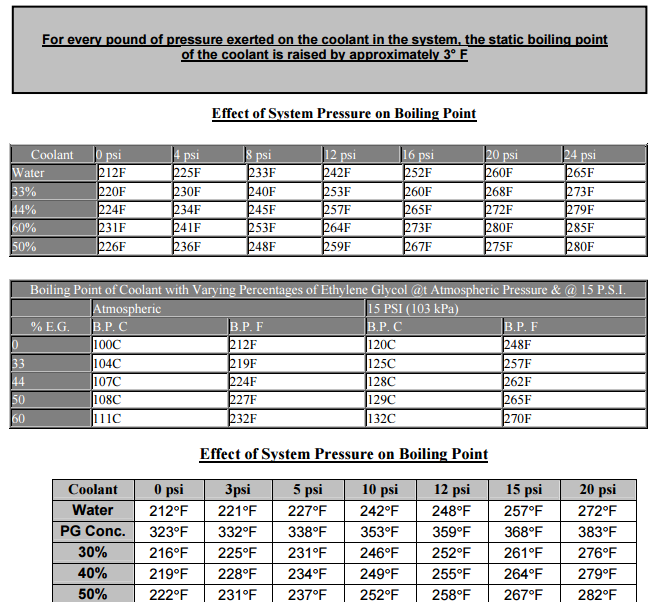
look thru, and read this linked info, the links help
adding an ANODE tends to slow the Corrosion, and having a good engine ground also tends to prevent or at least delay freeze plug and radiator corrosion problems running just water in a radiator allows it to act like the acid in a battery if your running aluminum parts like water pumps,radiators and heads on an iron block.
NEVER MIX
DEXCOOL (ORANGE)AND PRESTONE (GREEN)DON,T PLAY WELL TOGETHER
read thru the links
http://www.aa1car.com/library/2005/us90554.htm
http://www.radiator.com/article-radiator-coolant.html
http://www.aa1car.com/library/2004/us120426.htm
["quote=gch"]After several hot Texas summers, my 454 aluminum radiator has sprung a small leak. It's an Al core with plastic end caps. I'm replacing it. [color]Is an entire all Al radiator worth the price?[/color] I'm sure I can find a size close enough to fit my C10. Boiling over is an issue too. Should I rewire my electric fans to keep running with the key off until temp drop to 190? I don't like constant power to components, but a cut off switch would be helpful during cooler months. [/quote]
the value of an aftermarket radiator depends on the ratio of COST to quality and expected life span, the BETTER QUALITY aluminum 3 & 4 tube aluminum radiators do a very effective job at heat transfer and in my opinion are well worth the cost., theres a ton of related info in the links below

 www.forbes.com
www.forbes.com

 mechanicbase.com
mechanicbase.com

 www.carbibles.com
www.carbibles.com

 www.thetruthaboutcars.com
www.thetruthaboutcars.com

 www.thedrive.com
www.thedrive.com
 www.valvoline.com
www.valvoline.com
adding an ANODE can help, reduce corrosion .
http://www.radiator.com/article-radiator-problems.html
http://www.radiatorinfo.com/radtip.html
Antifreeze shenanigans
Last night I had a long discussion with some friends on antifreeze. I was amazed how many opposite opinions I heard on something that should have a pretty straight answer. Ethylene, propylene, organic, inorganic, silicates, long life, distilled, non-distilled, bolt in zinc anodes andwhatnot...
garage.grumpysperformance.com
http://www.aa1car.com/library/cooling_s ... rosion.htm
viewtopic.php?f=57&t=9769
http://www.sancarlosradiator.com/antifreeze_coolant.htm
http://forum.grumpysperformance.com/viewtopic.php?f=57&t=74
http://www.ehow.com/how_2189727_prevent-clogged-radiator.html
http://automechanics.wordpress.com/2008/05/28/top-ten-common-radiator-problems-for-older-vehicles/
the ORANGE and GREEN anti-freeze most of us use are sometimes NOT compatible and if blended , IF mixed they frequently over time form a sludge, or break down and leave crud in the radiator passages that clogs the passages, THEY SHOULD NEVER BE MIXED
https://durathermfluids.com/pdf/techpapers/pressure-boiling-point.pdf


look thru, and read this linked info, the links help
adding an ANODE tends to slow the Corrosion, and having a good engine ground also tends to prevent or at least delay freeze plug and radiator corrosion problems running just water in a radiator allows it to act like the acid in a battery if your running aluminum parts like water pumps,radiators and heads on an iron block.
NEVER MIX
DEXCOOL (ORANGE)AND PRESTONE (GREEN)DON,T PLAY WELL TOGETHER
read thru the links
http://www.aa1car.com/library/2005/us90554.htm
http://www.radiator.com/article-radiator-coolant.html
http://www.aa1car.com/library/2004/us120426.htm
["quote=gch"]After several hot Texas summers, my 454 aluminum radiator has sprung a small leak. It's an Al core with plastic end caps. I'm replacing it. [color]Is an entire all Al radiator worth the price?[/color] I'm sure I can find a size close enough to fit my C10. Boiling over is an issue too. Should I rewire my electric fans to keep running with the key off until temp drop to 190? I don't like constant power to components, but a cut off switch would be helpful during cooler months. [/quote]
the value of an aftermarket radiator depends on the ratio of COST to quality and expected life span, the BETTER QUALITY aluminum 3 & 4 tube aluminum radiators do a very effective job at heat transfer and in my opinion are well worth the cost., theres a ton of related info in the links below

Best Antifreeze and Coolant Fluids for 2022
Antifreeze prevents engine cooling fluids from freezing in winter and helps cool the engine in summer. Here are the best antifreeze and coolant fluids for 2022.

The 10 Best Coolants and Antifreezes
Find out how to choose the best engine coolant and antifreeze for your car based on factors such as quality, price, performance, warranty and more.

The Best Antifreeze (Review) in 2022
Car's cooling system is vital for a smooth engine performance. Check out our detailed guide about the best antifreezes on the market to save you time and money.
 www.carbibles.com
www.carbibles.com

Best Antifreeze: That's Cool
Antifreeze, or coolant, is vital to making sure your car runs properly. Check out the best antifreeze at The Truth About Cars.

Best Antifreeze: Keep Your Engine Cooler for Longer
Looking for antifreeze? Our team of experts narrowed down the best antifreeze on the market. Read this review and save yourself time and money.
Choose the Right Engine Coolant - Valvoline™ Global
Learn about the different types of engine coolant and make sure you're putting the right coolant in your vehicle. Valvoline's antifreeze color chart can help!
thoughts on cooling
read this, yes theres a good deal of info in the links worth the time and effort, and yes its well worth your time to read thru keep in mind, that if your engine is mechanically functioning correctly, IE there are no problems like leaking head gaskets or the wrong head gasket or a cracked head...
garage.grumpysperformance.com
anti freeze red vs green
ANY ANTI FREEZE needs to be replaced every 3-5 years or so or at 150K miles ,AS A MAXIMUM LIFE EXPECTANCY, MOST LOSE SIGNIFICANT LEVELS OF EFFECTIVE CORROSION PROTECTION GRADUALLY FROM 24 MONTHS and about 40,000 miles and its a good idea to have the RADIATOR FLUSHED of the crud that tends to...
garage.grumpysperformance.com
14 Rules for Improving Engine Cooling System Capability
I thought this article explained very well why slowing down the flow of coolant by using a restriction is wrong. Also goes a long ways to uncovering many of the myths that surround a cooling system. Mr Crook gives us some hard numbers that can be used when picking a radiator, such as the number...
garage.grumpysperformance.com
anodes
I got asked if USING anodes, in an engine is a good idea? electrolysis can rapidly cause damage, use of a 50% anti-freeze/50% water coolant mix and at least two good separate engine grounds to the cars frame helps but get out a multi meter and test for voltage in coolant, (more info in links)...
garage.grumpysperformance.com
Last edited by a moderator:





