ethanol has come back in a big way, with many race cars making the switch and even some street vehicles running the stuff. This begs the question: What is it about methanol that gives it an edge over gasoline?
keep in mind if the engines designed to run gas , its not maximizing its ethanol or methanol, alcohol power potential , and alcohol is very corrosive to most synthetics, plastics, and over time even aluminum
A popular discussion among fuel enthusiasts is how energy-dense a particular fuel is. While that’s important when discussing engine efficiency or brake specific fuel consumption (BSFC), a much more important question when discussing power output is the fuel’s maximum-power air/fuel ratio. For instance, in a gasoline engine, an air-to-fuel ratio of roughly 12.5:1 (12.5 parts air to 1 part fuel) is about right for maximum engine power. In the case of methanol, an air-to-fuel ratio of 4:1(4 parts air to 1 part fuel) is considered slightly on the rich side, but will allow the engine to make its maximum power.
While it’s true that gasoline has a higher energy density (about 18,400 BTU/pound) than methanol (9,500 BTU/pound), if you can burn three times more methanol than gasoline per power stroke, you can make more power. An engine that flows 1,000 cfm of air (about 70 pounds worth) means that on gasoline, the engine will consume about 5.6 pounds of fuel based upon its 12.5:1 max power ratio, giving a total energy output of (5.6 pounds x 18,400 BTU) or 103,040 BTUs of energy. If we do the same calculation on methanol, we get 17.5 pounds of fuel burned, and (17.5 pounds x 9,500 BTU) or 166,250 BTUs of energy—that’s a 60 percent greater energy output.
https://www.bellperformance.com/blog/4-things-you-need-to-know-about-ethanol-fuel
https://www.holley.com/products/fue...etering_block_conversion_kits/parts/34-106QFT
https://www.holley.com/products/fue...ering_block_conversion_kits/parts/34-45E85QFT
https://www.motortrendondemand.com/detail/gas-vs-e85-power-and-pros-cons/0_6hft5qjw/
https://petroclear.com/resources/dont-be-phased.php
https://en.wikipedia.org/wiki/Methanol_fuel
http://www.afdc.energy.gov/fuels/emerging_methanol.html
http://www.smokemup.com/tech/fuels.php
http://www.turbofast.com.au/racefuel2.html
http://www.hotrod.com/how-to/engine/ctrp-1201-alcohol-fuel-basics/
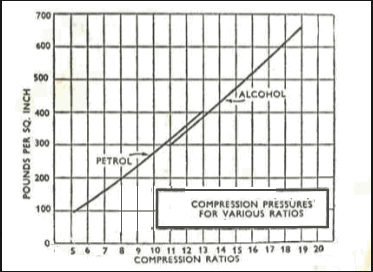
most efficient compression ratio for methanol alcohol fuel, is near 16:1
As a rough guide the ratio for petrol/gas is limited to about ten to one, or with certain additives to as much as 12 to one. With alcohol, however, you can go up to 19 to one or higher in certain cases. (For all practical purposes however, 14 to one should be considered the maximum usable ratio in modern short stroke automotive engines.)
At high engine loads, direct injection of highly knock-resistant methanol fuel offers a turbocharged, high-compression stoichiometric fuel/air ratio and substantial efficiency beyond conventional gasoline engines (around 30 percent).
Methanol occurs naturally in the human body and in some fruits, but is poisonous in high concentration. Ingestion of 10 ml can cause blindness and 60-100 ml can be fatal if the condition is untreated.[15] Like many volatile chemicals, methanol does not have to be swallowed to be dangerous since the liquid can be absorbed through the skin, and the vapors through the lungs. Methanol fuel is much safer when blended with ethanol, even at relatively low ethanol percentages.
Ethanol is currently (as of 2007) priced at 3 to 4 dollars per gallon (0.8 to 1.05 dollars per liter) at the pump, the expanding use of ethanol driven by producers of corn, while methanol made from natural gas remains at 47 cents per gallon (12.5 cents per liter) in bulk, not at the pump.
https://www.sunocoracefuels.com/tech-article/alcohol-and-octane
Benefits
The benefits of ethanol relative to race fuel and/or methanol are multifold, but let’s start with the one many of you already have guessed-power. A typical (R+M)/2 octane rating of E85 is around 100. The naturally high octane allows for greater compression and expansion ratios-the power and efficiency benefits to the racer of higher octane are well known and widely published.
Second, ethanol has a higher heat of vaporization relative to gasoline-a significantly higher value. A typical heat of vaporization for gasoline may be on the order of 59 kilojoules per kilogram of fuel. For ethanol, it’s approximately 130 kilojoules per kilogram, which is more than twice that value for gasoline.
Why is this important? Before a fuel combusts, it must exist as vapor mixed with air, using oxygen in the atmosphere as the source of its oxidant. Therefore, as liquid fuel is introduced into the manifold either by carburetion or fuel injection, it must first change from the liquid to vapor phase and sufficiently mix with the air before it will combust.
Compression makes horsepower on any fuel.
Methanol makes more power by providing more heat.
Gasoline at 20,943 BTU’s/lb at 14.7:1 air/fuel ratio by mass equals 0.068 lbs of fuel/lb of air or 1415 BTU’s/lb of air.
Methanol at 9,770 BTU’s/lb at 6.4 air/fuel ratio by mass equals 1527 BTU’s/lb of air. About 22% more bang.
Nitromethane at 5,160 BTU’s/lb at 1.7:1 air/fuel ratio by mass equals 3035 BTU’s/lb of air.
Methanol is commonly used “straight” – that's why it's called racing alcohol by many. Ethanol can also be used straight, and some racers do, but it's more common to hear about E85, a blend of about 85% ethanol. Much has been said about the octane rating of alcohols.
keep in mind if the engines designed to run gas , its not maximizing its ethanol or methanol, alcohol power potential , and alcohol is very corrosive to most synthetics, plastics, and over time even aluminum
A popular discussion among fuel enthusiasts is how energy-dense a particular fuel is. While that’s important when discussing engine efficiency or brake specific fuel consumption (BSFC), a much more important question when discussing power output is the fuel’s maximum-power air/fuel ratio. For instance, in a gasoline engine, an air-to-fuel ratio of roughly 12.5:1 (12.5 parts air to 1 part fuel) is about right for maximum engine power. In the case of methanol, an air-to-fuel ratio of 4:1(4 parts air to 1 part fuel) is considered slightly on the rich side, but will allow the engine to make its maximum power.
While it’s true that gasoline has a higher energy density (about 18,400 BTU/pound) than methanol (9,500 BTU/pound), if you can burn three times more methanol than gasoline per power stroke, you can make more power. An engine that flows 1,000 cfm of air (about 70 pounds worth) means that on gasoline, the engine will consume about 5.6 pounds of fuel based upon its 12.5:1 max power ratio, giving a total energy output of (5.6 pounds x 18,400 BTU) or 103,040 BTUs of energy. If we do the same calculation on methanol, we get 17.5 pounds of fuel burned, and (17.5 pounds x 9,500 BTU) or 166,250 BTUs of energy—that’s a 60 percent greater energy output.


https://www.bellperformance.com/blog/4-things-you-need-to-know-about-ethanol-fuel
https://www.holley.com/products/fue...etering_block_conversion_kits/parts/34-106QFT
https://www.holley.com/products/fue...ering_block_conversion_kits/parts/34-45E85QFT
https://www.motortrendondemand.com/detail/gas-vs-e85-power-and-pros-cons/0_6hft5qjw/
https://petroclear.com/resources/dont-be-phased.php
https://en.wikipedia.org/wiki/Methanol_fuel
http://www.afdc.energy.gov/fuels/emerging_methanol.html
http://www.smokemup.com/tech/fuels.php
http://www.turbofast.com.au/racefuel2.html
http://www.hotrod.com/how-to/engine/ctrp-1201-alcohol-fuel-basics/
most efficient compression ratio for methanol alcohol fuel, is near 16:1
As a rough guide the ratio for petrol/gas is limited to about ten to one, or with certain additives to as much as 12 to one. With alcohol, however, you can go up to 19 to one or higher in certain cases. (For all practical purposes however, 14 to one should be considered the maximum usable ratio in modern short stroke automotive engines.)
At high engine loads, direct injection of highly knock-resistant methanol fuel offers a turbocharged, high-compression stoichiometric fuel/air ratio and substantial efficiency beyond conventional gasoline engines (around 30 percent).
Methanol occurs naturally in the human body and in some fruits, but is poisonous in high concentration. Ingestion of 10 ml can cause blindness and 60-100 ml can be fatal if the condition is untreated.[15] Like many volatile chemicals, methanol does not have to be swallowed to be dangerous since the liquid can be absorbed through the skin, and the vapors through the lungs. Methanol fuel is much safer when blended with ethanol, even at relatively low ethanol percentages.
Ethanol is currently (as of 2007) priced at 3 to 4 dollars per gallon (0.8 to 1.05 dollars per liter) at the pump, the expanding use of ethanol driven by producers of corn, while methanol made from natural gas remains at 47 cents per gallon (12.5 cents per liter) in bulk, not at the pump.
https://www.sunocoracefuels.com/tech-article/alcohol-and-octane
Benefits
The benefits of ethanol relative to race fuel and/or methanol are multifold, but let’s start with the one many of you already have guessed-power. A typical (R+M)/2 octane rating of E85 is around 100. The naturally high octane allows for greater compression and expansion ratios-the power and efficiency benefits to the racer of higher octane are well known and widely published.
Second, ethanol has a higher heat of vaporization relative to gasoline-a significantly higher value. A typical heat of vaporization for gasoline may be on the order of 59 kilojoules per kilogram of fuel. For ethanol, it’s approximately 130 kilojoules per kilogram, which is more than twice that value for gasoline.
Why is this important? Before a fuel combusts, it must exist as vapor mixed with air, using oxygen in the atmosphere as the source of its oxidant. Therefore, as liquid fuel is introduced into the manifold either by carburetion or fuel injection, it must first change from the liquid to vapor phase and sufficiently mix with the air before it will combust.
Compression makes horsepower on any fuel.
Methanol makes more power by providing more heat.
Gasoline at 20,943 BTU’s/lb at 14.7:1 air/fuel ratio by mass equals 0.068 lbs of fuel/lb of air or 1415 BTU’s/lb of air.
Methanol at 9,770 BTU’s/lb at 6.4 air/fuel ratio by mass equals 1527 BTU’s/lb of air. About 22% more bang.
Nitromethane at 5,160 BTU’s/lb at 1.7:1 air/fuel ratio by mass equals 3035 BTU’s/lb of air.
Methanol is commonly used “straight” – that's why it's called racing alcohol by many. Ethanol can also be used straight, and some racers do, but it's more common to hear about E85, a blend of about 85% ethanol. Much has been said about the octane rating of alcohols.
Last edited:
