theres a long list of factors that have to be considered and balanced for the internal combustion engine to make better power
,one major factor is the type of fuel used and the fuel/air ratio and its efficient combustion, if you want to boost power you need to more effectively increase combustion efficiency, you can use a super charger or turbo to force a greater volume of fuel/air mix into the combustion chambers thus boosting pressures on the power stroke ,or you can change the fuel, to prolong the combustion and pressure above the piston.
fuel like alcohol, can be used that allows a much greater volume of slightly less powerful fuel to be burnt over a slightly longer time frame at a higher compression ratio, this can easily boost power 10%-15% over a rather similar gas engine , PROVIDED changes required to maximize alcohols burn characteristics are maximized.
and YES YOU CAN USE NITROUS , WITH ITS INCREASED OXYGEN CONTENT WITH ALCOHOL , WITH ITS GREATER VOLUM OF FUEL<
to maximize both the burn and prolonged burn/pressure characteristics of both when properly mixed in the correct ratio and used with higher compression.
ok, the basics, it takes about 50 thousands of a second at lower rpms ,
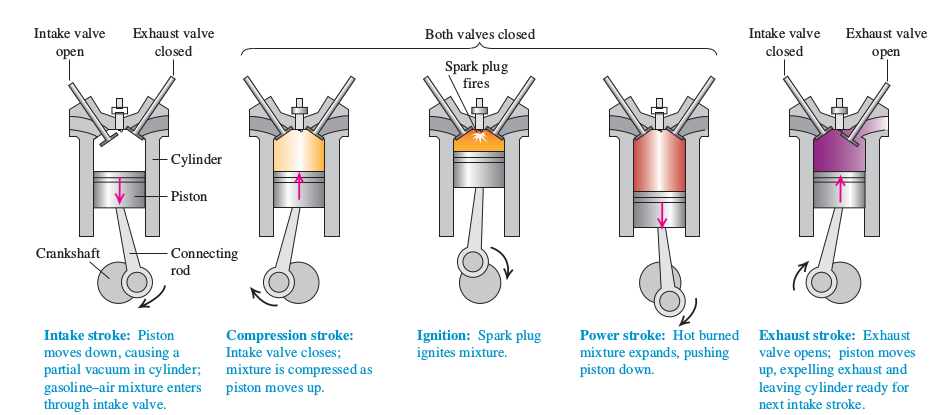
for your typical 4 stroke gas engine for the fuel/air mix thats being compressed to ignite and burn,
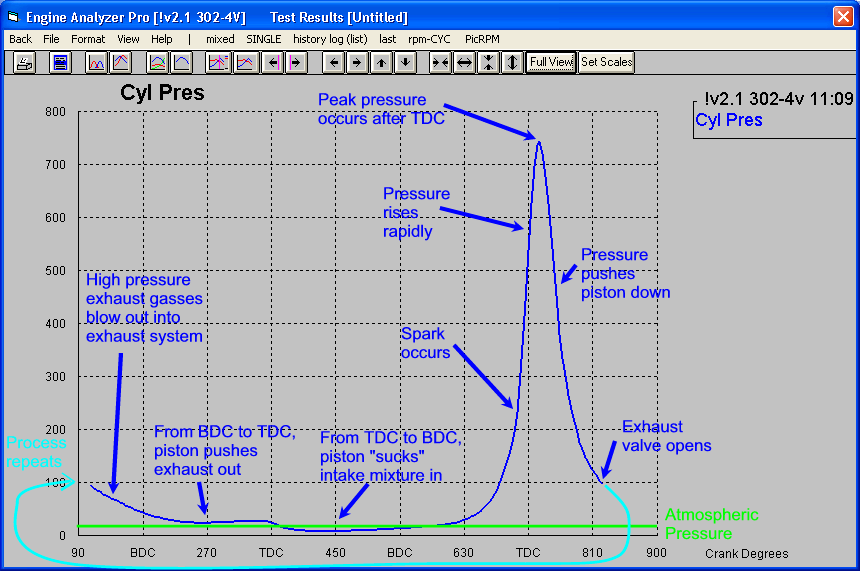
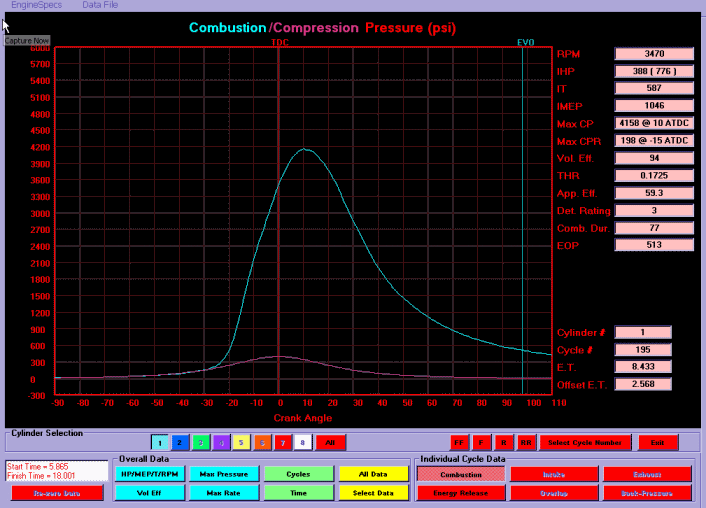
producing pressure above the piston, that peak pressure is reached, peaks and is dropping rapidly by the time the piston is less than 30 degrees past TDC., at idle, lets say at 750 rpm, theres 12.5 crankshaft revolutions per second,
thats 6.25 power strokes per second, and each revolution is 360 degrees, or 1/12th of that power stroke is actually producing power,
thus either increasing pressure or finding a way to produce a longer duration pressure pulse above the piston with a greater volume of fuel that burns a bit longer tends to increase power.
each revolution at idle tales about .16 of a second , so about .013 seconds is used to produce power,
now consider theres a 720 degree repetitive cycle, and that means that less than 1/24th of that 720 degrees is really producing power.
or put a bit differently peak piston pressure lasts from just after TDC to only about 25-30 degrees past TDC, where pressure drops off rapidly.
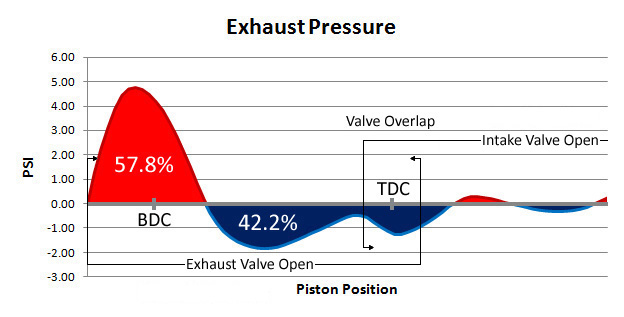
and the slower the engine rotates the more of that pressure is NEGATIVE WORK, in that its building up over the piston BEFORE it reaches TDC thus reducing efficiency, now cylinder head design and the cam timing compression ratio and fuel/air ratio all effect efficiency, and above about 4500 rpm the increased speed of piston compression and port flow interacting with exhaust SCAVENGING can and usually does help increase the flame front speed and combustion efficiency and any back pressure tends to reduce scavenging efficiency/ thus power output.
exhaust SCAVENGING, helps cylinder fill, the cylinder more efficiently with a better fuel/air ratio , as the inertia of the previous combustion gases exiting, the cylinder, helps draw in the next intake runners mass.
AS ALWAYS READ THE LINKED INFO

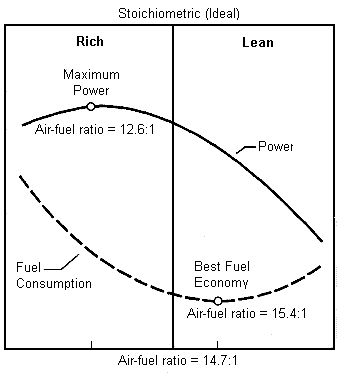
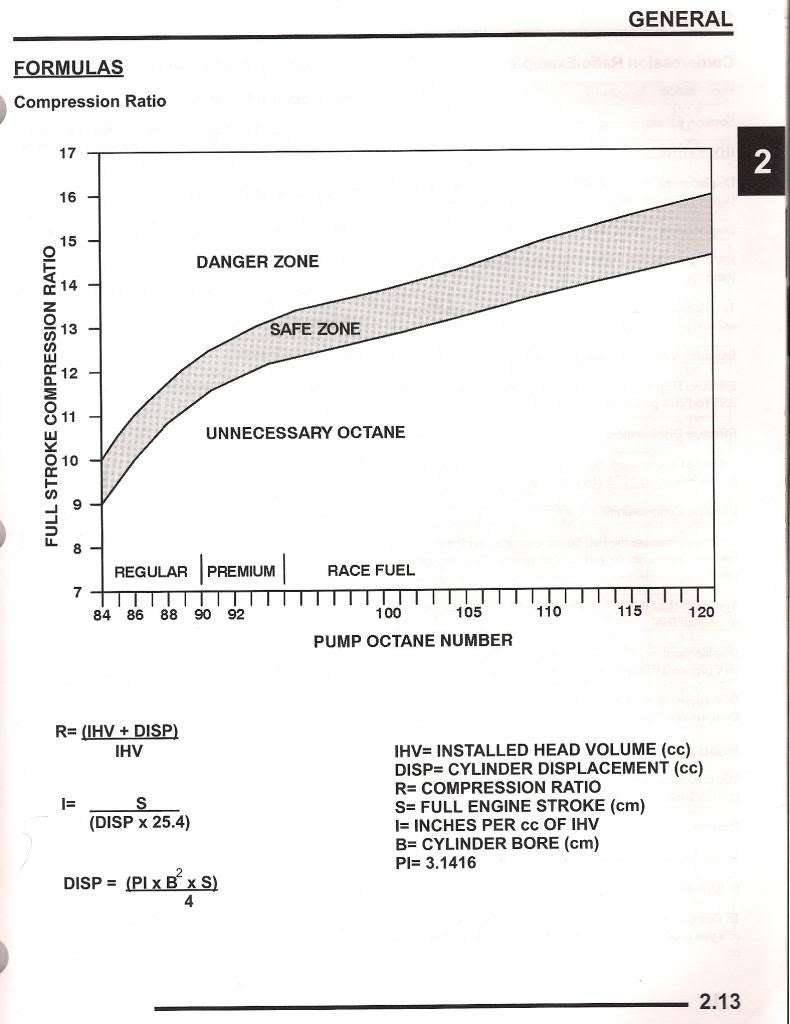
gas engines run most efficiently with about a 12.7:1 fuel/air ratio, and ideally about a 12.5:1 compression ratio, with again, ideally about a 103 octane fuel.
If your thinking of running pump fuel, the simple answer..
try to keep your dynamic compression ratio at 8:1,or lower, your intake air temp as low as possible,your oil temperature below about 220f and your coolant temp below about 190f and use 92-or higher octane fuel, and use an ignition system with a knock sensor if possible
READ THRU THE LINKS ITS WELL WORTH THE EFFORT
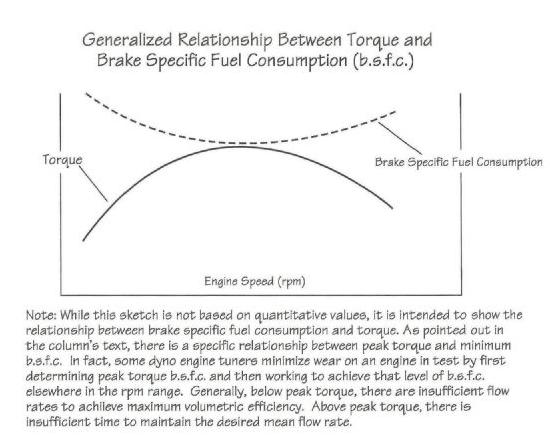
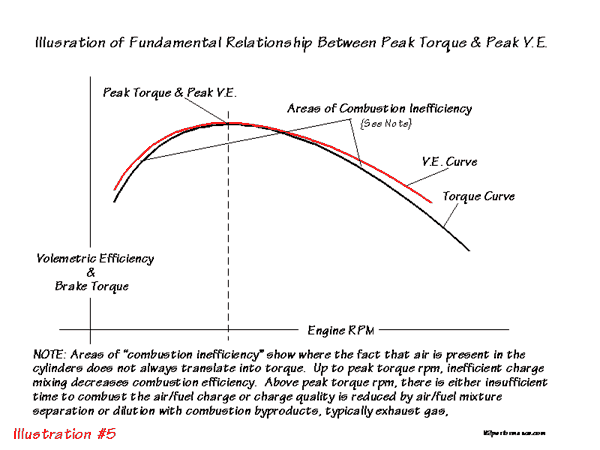
that

keep in mind changing the fuel and oxygen content of the mix, can have a pronounced effect on that pressure curve and its duration,
example
if you use NITROUS, you have changed the oxygen content of the (air) from a 21% oxygen content to about a 40% oxygen content so at least in theory you could potentially DOUBLE the engine power output as the amount of fuel burnt in each combustion cycle can in theory be almost double what it could be using strait atmospheric (AIR).
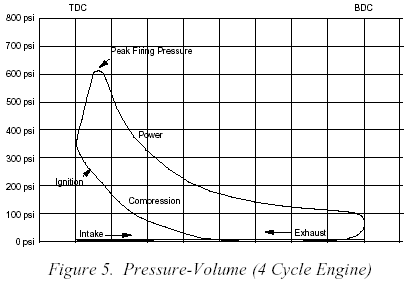
Peak cylinder pressure (PCP)is the maximum chamber pressure achieved during the combustion process. This figure would normally be in the 600 to 2000 psi range. Thermal efficiency describes the amount of energy extracted to perform useful work from the total energy contained in the fuel.Feb 29, 2000
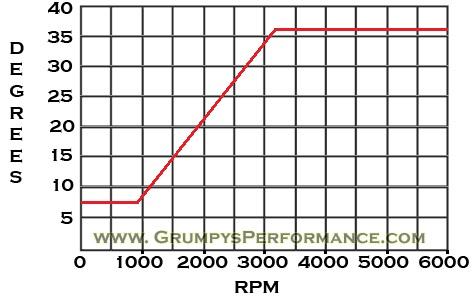
it would make sense that a highly compressed mix of alcohol laced petroleum (LIKE E85) ,(not a great electrical conductor, but a reasonably good heat absorbing mass) of molecules, even though its gaining heat through compression, and that alcohol/air and petroleum mix of compressed atmosphere gases, which must be moving rapidly moving, and being heated by mechanical, compression, as its being compressed and forced into an ever shrinking and thus a smaller/denser area, thus packed rather densely would require a smaller gap so that the electrical arc between the spark plug electrode and ground strap would require both higher volts to jump the gap and more amps to build the required thermal heat to ignite the gaseous mix that might be effectively at least partially insulating the plugs electrical gap, there's roughly a .50 thousands of a second, time frame between ignition and the flame front crossing the cylinder, at least up to about 3000 rpm, once the rpms exceed the approximate 3000 rpm level the combination of rapid compression and turbulence starts to increase the burn speed, that's why you don't need to keep increasing the ignition advance on the distributor past the 3000 rpm and the ever shorter time due to the faster rpms compensates.
(one reason E85 is less prone to detonation issues is ethanol alcohol is harder to ignite due to compression heat)
keep in mind at idle, (lets say 750 rpm)(remember every other time the piston hits tdc is the compression and ignition cycle) thus at 750 rpm you need an ignition electrical arc 6.2 times per second at the spark plug, for the flame front to have time to fully ignite the cylinder, at idle but by 7000 rpm you need roughly 58 times a second.
remember any pressure over the piston before TDC reduces power, and you make power with pressure above the piston as it travels down forcing the crank through the connecting rod on the crank journal , but the pressure drops rapidly as the volume , of burning gases providing that pressure above the piston increases, and the fuel/air mix burns out.
obviously the type of fuel and the volume of fuel effects the burn characteristics
typical gas burns out before the piston has dropped more than about 25-30 degrees
nitro methane burns longer and provides more total pressure per gram of fuel so it continues to burn to about 50 degrees past TDC.
2. COMBUSTION QUALITY EVALUATION FOR SPARK IGNITION ENGINES
The combustion of spark ignition engines can be divided into three frames: ignition
and flame development; flame propagation; flame termination. Flame development is
generally considered the consumption of the first 5% - 10% of the combustible mixture.
During the flame development period, ignition occurs and the combustion process starts,
but very little pressure rise is noticeable and little or no useful work is produced (Fig. 3).
Just about all useful work produced in an engine cycle is the result of the flame
propagation period of then combustion process. This is the period when the bulk of the
fuel and air mass is burned (80-90%). During this time, pressure in the cylinder is greatly
increased, and this provides the force to produce work in the expansion stroke. The final
5% - 10% of the mixture which burns is classified as flame termination. During this time,
pressure quickly decreases and combustion stops [5].
2. COMBUSTION QUALITY EVALUATION FOR SPARK IGNITION ENGINES
The combustion of spark ignition engines can be divided into three frames: ignition
and flame development; flame propagation; flame termination. Flame development is
generally considered the consumption of the first 5% - 10% of the combustible mixture.
During the flame development period, ignition occurs and the combustion process starts,
but very little pressure rise is noticeable and little or no useful work is produced (Fig. 3).
Just about all useful work produced in an engine cycle is the result of the flame
propagation period of then combustion process. This is the period when the bulk of the
fuel and air mass is burned (80-90%). During this time, pressure in the cylinder is greatly
increased, and this provides the force to produce work in the expansion stroke. The final
5% - 10% of the mixture which burns is classified as flame termination. During this time,
pressure quickly decreases and combustion stops [5].
FIG. 3. Theoretical cylinder pressure curve for spark ignition (SI) engines
Theoretically, combustion would be exactly the same for every engine cycle, and
there would be no cycle-to-cycle variation. This does not happen due to several variations
that occur in the intake system and within the cylinder. Even if no variations occurred
before combustion, the turbulence within the cylinder would cause statistical variations to
occur during combustion. Temperature differences in the runners cause variations in the
evaporation rates, and this causes variations in the air-fuel ratio. More fuel vapor in a
hotter runner will displace more air and give a richer mixture and lower volumetric
efficiency. Also, the evaporative cooling causes temperature differences and density
differences. Passage of air around the throttle plate breaks into two flows, causing
vortices and other variations that will then affect all downstream flow [6].
Local variations and incomplete mixing, near the spark plug, cause the initial
discharge across the electrodes to vary from the average, which then initiates combustion
differently cycle-to-cycle. Once there is a difference in the start of combustion, the entire
following combustion process will be changed. Figure 4 shows how pressure varies as a

 www.researchgate.net
www.researchgate.net

 www.enginebuildermag.com
www.enginebuildermag.com

 www.motortrend.com
www.motortrend.com
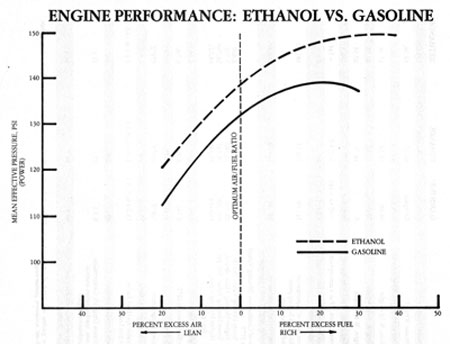
Methanol/ ETHANOL alcohol does not have as much power per ounce as gasoline but its power potential is higher because it can be burn ay considerably higher compression ratios and you can burn it at a considerably higher fuel to air ratio.
Stoich for gasoline is ~14.7:1. Stoich for E85 is roughly 9.7:1 --- this means you need more E85 for every part air taken into the engine - roughly 35% more (i.e. 35% drop in MPG).
Methanol has long been a popular racing fuel because of its increased power potential. Methanol is widely available and can be made from natural gas, coal or other feedstocks. Its chemical formula is CH3OH. It contains oxygen and is relatively inexpensive as far as fuels go.
But it contains only about half the BTU content of gasoline (64,600 BTUs/gallon) so it requires a much richer air/fuel mixture and a higher flow capacity fuel system (larger carburetor jets, high volume fuel pump and/or higher flow fuel injectors). Methanol likes RICH fuel mixtures (from 4.5 to 1 to 6.0 to 1 for peak power) so it takes twice as much methanol to produce the same power as gasoline.
Methanol has a high octane rating: 129 RON, 103 MON, or a pump AKI rating of 113. Because of its higher octane rating (which allows more compression) and the added oxygen content, the overall power gain with methanol can be 10% to 20% or more over pump gas depending on the compression ratio and how the carburetor is jetted.
The purity of methanol can vary depending on where it is sourced and how it is handled so some suppliers certify the purity of their methanol racing fuel to guarantee consistent performance. Methanol is generally sold in sealed steel containers and should be kept in sealed steel containers for safety purposes. Methanol is corrosive so it requires stainless steel fuel lines and a fuel tank with a methanol-compatible bladder. Methanol is also toxic (it will kill you if you drink it) and produces nasty exhaust fumes.
nitro-methane
 www.sciencedirect.com
www.sciencedirect.com
 x-engineer.org
x-engineer.org
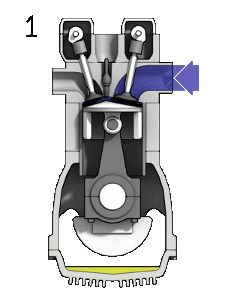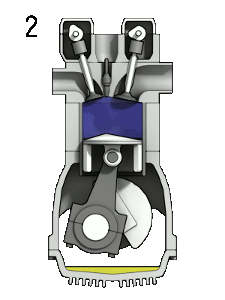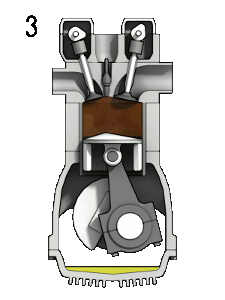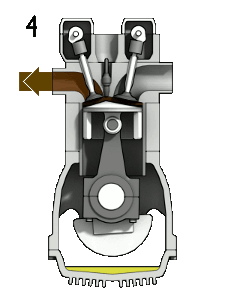
 energyeducation.ca
energyeducation.ca
,one major factor is the type of fuel used and the fuel/air ratio and its efficient combustion, if you want to boost power you need to more effectively increase combustion efficiency, you can use a super charger or turbo to force a greater volume of fuel/air mix into the combustion chambers thus boosting pressures on the power stroke ,or you can change the fuel, to prolong the combustion and pressure above the piston.
fuel like alcohol, can be used that allows a much greater volume of slightly less powerful fuel to be burnt over a slightly longer time frame at a higher compression ratio, this can easily boost power 10%-15% over a rather similar gas engine , PROVIDED changes required to maximize alcohols burn characteristics are maximized.
and YES YOU CAN USE NITROUS , WITH ITS INCREASED OXYGEN CONTENT WITH ALCOHOL , WITH ITS GREATER VOLUM OF FUEL<
to maximize both the burn and prolonged burn/pressure characteristics of both when properly mixed in the correct ratio and used with higher compression.
ok, the basics, it takes about 50 thousands of a second at lower rpms ,
for your typical 4 stroke gas engine for the fuel/air mix thats being compressed to ignite and burn,
producing pressure above the piston, that peak pressure is reached, peaks and is dropping rapidly by the time the piston is less than 30 degrees past TDC., at idle, lets say at 750 rpm, theres 12.5 crankshaft revolutions per second,
thats 6.25 power strokes per second, and each revolution is 360 degrees, or 1/12th of that power stroke is actually producing power,
thus either increasing pressure or finding a way to produce a longer duration pressure pulse above the piston with a greater volume of fuel that burns a bit longer tends to increase power.
each revolution at idle tales about .16 of a second , so about .013 seconds is used to produce power,
now consider theres a 720 degree repetitive cycle, and that means that less than 1/24th of that 720 degrees is really producing power.
or put a bit differently peak piston pressure lasts from just after TDC to only about 25-30 degrees past TDC, where pressure drops off rapidly.
and the slower the engine rotates the more of that pressure is NEGATIVE WORK, in that its building up over the piston BEFORE it reaches TDC thus reducing efficiency, now cylinder head design and the cam timing compression ratio and fuel/air ratio all effect efficiency, and above about 4500 rpm the increased speed of piston compression and port flow interacting with exhaust SCAVENGING can and usually does help increase the flame front speed and combustion efficiency and any back pressure tends to reduce scavenging efficiency/ thus power output.
exhaust SCAVENGING, helps cylinder fill, the cylinder more efficiently with a better fuel/air ratio , as the inertia of the previous combustion gases exiting, the cylinder, helps draw in the next intake runners mass.
AS ALWAYS READ THE LINKED INFO

calculating header design
YES THE SUB LINK INFO IS WELL WORTH READING ALSO if your serious about maintaining good peak hp numbers use the calculators in the linked threads to actually calculate the ideal matched header dimensions, this is not a guessing game its strait physics and easily calculated to maximize exhaust...
garage.grumpysperformance.com


gas engines run most efficiently with about a 12.7:1 fuel/air ratio, and ideally about a 12.5:1 compression ratio, with again, ideally about a 103 octane fuel.
If your thinking of running pump fuel, the simple answer..
try to keep your dynamic compression ratio at 8:1,or lower, your intake air temp as low as possible,your oil temperature below about 220f and your coolant temp below about 190f and use 92-or higher octane fuel, and use an ignition system with a knock sensor if possible
READ THRU THE LINKS ITS WELL WORTH THE EFFORT




that

keep in mind changing the fuel and oxygen content of the mix, can have a pronounced effect on that pressure curve and its duration,
example
if you use NITROUS, you have changed the oxygen content of the (air) from a 21% oxygen content to about a 40% oxygen content so at least in theory you could potentially DOUBLE the engine power output as the amount of fuel burnt in each combustion cycle can in theory be almost double what it could be using strait atmospheric (AIR).
Peak cylinder pressure (PCP)is the maximum chamber pressure achieved during the combustion process. This figure would normally be in the 600 to 2000 psi range. Thermal efficiency describes the amount of energy extracted to perform useful work from the total energy contained in the fuel.Feb 29, 2000
is backpressure hurting your combo?
any automotive engine will run best with little or no back pressure on the exhaust system , back pressure ALWAYS tends to reduce the cylinder fill and scavenging efficiency, this is not a guessing game. you can simply measure back pressure in the exhaust every500 rpm from about 3000rpm to where...
garage.grumpysperformance.com
it would make sense that a highly compressed mix of alcohol laced petroleum (LIKE E85) ,(not a great electrical conductor, but a reasonably good heat absorbing mass) of molecules, even though its gaining heat through compression, and that alcohol/air and petroleum mix of compressed atmosphere gases, which must be moving rapidly moving, and being heated by mechanical, compression, as its being compressed and forced into an ever shrinking and thus a smaller/denser area, thus packed rather densely would require a smaller gap so that the electrical arc between the spark plug electrode and ground strap would require both higher volts to jump the gap and more amps to build the required thermal heat to ignite the gaseous mix that might be effectively at least partially insulating the plugs electrical gap, there's roughly a .50 thousands of a second, time frame between ignition and the flame front crossing the cylinder, at least up to about 3000 rpm, once the rpms exceed the approximate 3000 rpm level the combination of rapid compression and turbulence starts to increase the burn speed, that's why you don't need to keep increasing the ignition advance on the distributor past the 3000 rpm and the ever shorter time due to the faster rpms compensates.
(one reason E85 is less prone to detonation issues is ethanol alcohol is harder to ignite due to compression heat)
keep in mind at idle, (lets say 750 rpm)(remember every other time the piston hits tdc is the compression and ignition cycle) thus at 750 rpm you need an ignition electrical arc 6.2 times per second at the spark plug, for the flame front to have time to fully ignite the cylinder, at idle but by 7000 rpm you need roughly 58 times a second.
remember any pressure over the piston before TDC reduces power, and you make power with pressure above the piston as it travels down forcing the crank through the connecting rod on the crank journal , but the pressure drops rapidly as the volume , of burning gases providing that pressure above the piston increases, and the fuel/air mix burns out.

obviously the type of fuel and the volume of fuel effects the burn characteristics
typical gas burns out before the piston has dropped more than about 25-30 degrees
nitro methane burns longer and provides more total pressure per gram of fuel so it continues to burn to about 50 degrees past TDC.


2. COMBUSTION QUALITY EVALUATION FOR SPARK IGNITION ENGINES
The combustion of spark ignition engines can be divided into three frames: ignition
and flame development; flame propagation; flame termination. Flame development is
generally considered the consumption of the first 5% - 10% of the combustible mixture.
During the flame development period, ignition occurs and the combustion process starts,
but very little pressure rise is noticeable and little or no useful work is produced (Fig. 3).
Just about all useful work produced in an engine cycle is the result of the flame
propagation period of then combustion process. This is the period when the bulk of the
fuel and air mass is burned (80-90%). During this time, pressure in the cylinder is greatly
increased, and this provides the force to produce work in the expansion stroke. The final
5% - 10% of the mixture which burns is classified as flame termination. During this time,
pressure quickly decreases and combustion stops [5].

2. COMBUSTION QUALITY EVALUATION FOR SPARK IGNITION ENGINES
The combustion of spark ignition engines can be divided into three frames: ignition
and flame development; flame propagation; flame termination. Flame development is
generally considered the consumption of the first 5% - 10% of the combustible mixture.
During the flame development period, ignition occurs and the combustion process starts,
but very little pressure rise is noticeable and little or no useful work is produced (Fig. 3).
Just about all useful work produced in an engine cycle is the result of the flame
propagation period of then combustion process. This is the period when the bulk of the
fuel and air mass is burned (80-90%). During this time, pressure in the cylinder is greatly
increased, and this provides the force to produce work in the expansion stroke. The final
5% - 10% of the mixture which burns is classified as flame termination. During this time,
pressure quickly decreases and combustion stops [5].
FIG. 3. Theoretical cylinder pressure curve for spark ignition (SI) engines
Theoretically, combustion would be exactly the same for every engine cycle, and
there would be no cycle-to-cycle variation. This does not happen due to several variations
that occur in the intake system and within the cylinder. Even if no variations occurred
before combustion, the turbulence within the cylinder would cause statistical variations to
occur during combustion. Temperature differences in the runners cause variations in the
evaporation rates, and this causes variations in the air-fuel ratio. More fuel vapor in a
hotter runner will displace more air and give a richer mixture and lower volumetric
efficiency. Also, the evaporative cooling causes temperature differences and density
differences. Passage of air around the throttle plate breaks into two flows, causing
vortices and other variations that will then affect all downstream flow [6].
Local variations and incomplete mixing, near the spark plug, cause the initial
discharge across the electrodes to vary from the average, which then initiates combustion
differently cycle-to-cycle. Once there is a difference in the start of combustion, the entire
following combustion process will be changed. Figure 4 shows how pressure varies as a

FIG. 3. Theoretical cylinder pressure curve for spark ignition (SI)...
Download scientific diagram | Theoretical cylinder pressure curve for spark ignition (SI) engines from publication: RESEARCHES ON COMBUSTION QUALITY FOR A GDI EXPERIMENTAL ENGINE | | ResearchGate, the professional network for scientists.

Fast Facts About Nitro Fuel - RC Car Action
RC Cars & Trucks | News | Traxxas Horizon Hobby Tamiya and more | Readers Ride PIT TIPS Nitro RCX Event Coverage | Fast Facts About Nitro Fuel
www.rccaraction.com
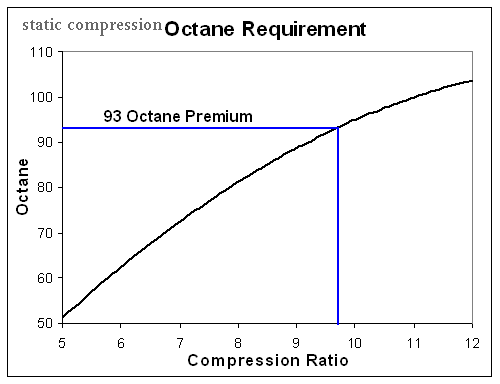
calculate required octane for compression ratio
What else influences your car's octane requirements? IF YOU DON,T LIKE READING SUB-LINKS, AND LOOKING AT CHARTS , AND DOING A BIT OF MATH 'AND INDIVIDUAL CALCULATIONS, YOUR UN-LIKELY TO BENEFIT FROM MOST OF THE THREADS, ON THIS WEB SITE! LIKE IN MOST AREAS ITS KNOWLEDGE AND THE ABILITY TO THINK...
garage.grumpysperformance.com
You want to make a high-power 4-stroke engine, there are a couple of different ways to accomplish your goal. One way is to increase the displacement. Another is to stuff more air into the engine with a turbocharger or a supercharger. If you want to go to extremes, you would replace gasoline altogether and use a more energetic fuel. Top fuel dragsters do all three. A "nitro-burning" engine and a "top fuel" engine are the same thing -- engines designed to burn nitromethane rather than gasoline. Gasoline is a hydrocarbon, and the common chemical formula for gasoline is C8H18. Nitromethane has the formula CH3NO2. Nitromethane is a little like gasoline that has been pre-mixed with nitrous oxide. The fuel comes with its own oxygen atoms to help it burn. The big advantage of nitromethane is that you can get a lot more power from each explosion inside the engine. Pound for pound, nitromethane is less energetic than gasoline, but you can burn a lot more nitromethane in a cylinder. The net result is more power per stroke. You typically need about 15 pounds of air to burn 1 pound of gasoline, whereas you need only 1.7 pounds of air to burn 1 pound of nitromethane. This means that, compared to gasoline, you can pump about 8 times more nitromethane into a cylinder of a given volume and still get complete combustion. Since nitromethane is not as dense as gasoline in terms of energy, you do not get an 8-time improvement in terms of power. It is more like a 2.5-time improvement (see this page for a comparison). Still, you can double or triple your engine's horsepower simply by changing the fuel. That's a huge improvement! A typical drag-racing engine has a displacement of 8.9 liters, is supercharged and produces about 6,000 horsepower. It can burn close to a gallon (4 liters) of nitromethane per second! To put that in perspective, there is something like 2 teaspoons (10 cc) of nitromethane being poured into each cylinder per intake stroke. An interesting thing about nitromethane is that it does not burn as quickly as gasoline. In fact, there is not enough time to burn all of the nitromethane between when the spark plug fires and when the exhaust valve opens. So the engine is pumping still-burning nitromethane into the exhaust pipe. That's why you see flames shooting out of the exhaust of a drag-racing car.
HPBG: The Power of Racing Fuels - Engine Builder Magazine
An engine’s power potential depends on how much air it breathes and the fuel it burns. Engine modifications such as larger bores and longer strokes add displacement, which increase airflow and power. Bigger valves, CNC ported heads and a hotter camshaft with increased lift and duration also...

What’s the cheapest way to increase horsepower? The answer is nitrous oxide.
Nitrous oxide is the least expensive way to add big power to your engine. Nitrous is safe so long as you understand how it works.
thinking about running E85 in your old muscle car?
69MyWay posted this info Don't do it..... You are going to kill your fuel tank, rubber, and metal lines...as well as the inner workings of the carb, that were never designed to work with E85 in those older cars, yes It will run fairly well in the short term, due to its high octane, but it...
garage.grumpysperformance.com
Methanol/ ETHANOL alcohol does not have as much power per ounce as gasoline but its power potential is higher because it can be burn ay considerably higher compression ratios and you can burn it at a considerably higher fuel to air ratio.
Stoich for gasoline is ~14.7:1. Stoich for E85 is roughly 9.7:1 --- this means you need more E85 for every part air taken into the engine - roughly 35% more (i.e. 35% drop in MPG).

Methanol has long been a popular racing fuel because of its increased power potential. Methanol is widely available and can be made from natural gas, coal or other feedstocks. Its chemical formula is CH3OH. It contains oxygen and is relatively inexpensive as far as fuels go.
But it contains only about half the BTU content of gasoline (64,600 BTUs/gallon) so it requires a much richer air/fuel mixture and a higher flow capacity fuel system (larger carburetor jets, high volume fuel pump and/or higher flow fuel injectors). Methanol likes RICH fuel mixtures (from 4.5 to 1 to 6.0 to 1 for peak power) so it takes twice as much methanol to produce the same power as gasoline.
Methanol has a high octane rating: 129 RON, 103 MON, or a pump AKI rating of 113. Because of its higher octane rating (which allows more compression) and the added oxygen content, the overall power gain with methanol can be 10% to 20% or more over pump gas depending on the compression ratio and how the carburetor is jetted.
The purity of methanol can vary depending on where it is sourced and how it is handled so some suppliers certify the purity of their methanol racing fuel to guarantee consistent performance. Methanol is generally sold in sealed steel containers and should be kept in sealed steel containers for safety purposes. Methanol is corrosive so it requires stainless steel fuel lines and a fuel tank with a methanol-compatible bladder. Methanol is also toxic (it will kill you if you drink it) and produces nasty exhaust fumes.
nitro-methane

Cylinder Pressure - an overview | ScienceDirect Topics
The pressure-volume (pV) diagram and how work is produced in an ICE – x-engineer.org
Tutorial on how the pressure volume (pV) diagram is produced and how work is generated in an internal combustion engine

Four stroke engine - Energy Education
Last edited:





